Pester
Possible Phase Separation Effects on the Na/Ca ratio in Mid-Ocean Ridge Hydrothermal Vent Fluids
N.J. Pester¹* & W.E. Seyfried, Jr.¹
Corresponding author: peste005@umn.edu
¹University of Minnesota, Department of Geology and Geophysics, Minneapolis, MN 55455
Abstract:
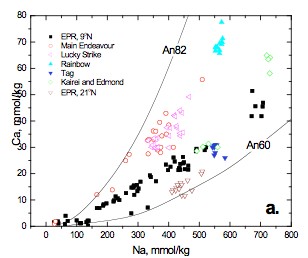
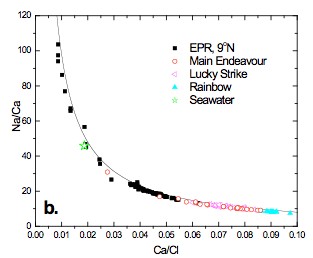
New data from the Lucky Strike (~37°N) and Rainbow (~36°N) hydrothermal fields on the Mid-Atlantic Ridge and East Pacific Rise (EPR) 9°N in 2008, in addition to the Main Endeavour Field (MEF), Juan de Fuca Ridge, in 2005, contribute to the time series data sets for these well characterized sites. Both Lucky Strike and MEF are basalt hosted systems located on intermediate spreading ridges. Furthermore, both have axial magma chambers (AMC) envisioned at ~3 km below the seafloor. EPR 9°N is a fast-spreading basalt hosted system with an AMC ~1.5 km below the seafloor, whereas Rainbow is slow-spreading ultramafic system. Data from these four systems represent well the broad spectrum of mid-ocean ridge hydrothermal systems, including time series data in the aftermath of an eruption and a major seismic event at EPR 9°N and MEF, respectively. Experiments are currently underway to assess the effects of near critical and two-phase behavior on the chemistry of high temperature fluids issuing from mid-ocean ridge hydrothermal systems. Phase separation in the NaCl-H2O system is widely accepted to account for the observed salinity deviations with respect to seawater (~6-200%) in these fluids. The Na/Ca concentration ratio in vent fluids is, to a first order, controlled by albite-anorthite solid solutions (Fig 1a. after Berndt and Seyfried, 1993) where, based on experimental data at 400°C and 400 bars, vent fluids seem to have equilibrated with quartz and plagioclase solid solutions of ~An60 to An70. The effects of variable temperature and density on these equilibria, however, have yet to be rigorously examined. Current and previous investigations demonstrate that with increasing distance from the critical curve in the two-phase region, the Na/Ca ratio increases in the vapor phase, while simultaneously decreasing in the liquid phase, relative to the homogenous (single-phase) fluid. A simple observation such as this may help explain observed trends in time-series chemistry from a variety of well studied vent fields. Ca/Cl vs. Na/Ca (Fig 1b.) exhibits a tight trend (based on charge balance) where the fast, intermediate and slow spreading systems occupy different regions of the curve. In the aftermath of a magmatic event, both EPR, 9°N and MEF exhibit the lowest salinities and the highest Na/Ca, whereas the highest salinities have the lowest Na/Ca. Na/Ca generally increases with increasing temperature and decreasing salinity (i.e. Na, Fig 1a.), parameters that are consistent with recent magmatic activity. It is, however, difficult to assess the role of Ca partitioning relative to Na driven fluid mineral equilibria during phase separation, especially since temperature effects under such conditions are not well constrained. Interestingly, Rainbow has the lowest Na/Ca, yet the fluids have high exit temperatures (~380°C), suggesting the high salinity of these fluids may play a larger role.
Contributions to Integration and Synthesis:
There is a dearth of experimental data regarding metal transport and fluid-mineral phase equilibria at the critical temperatures and pressures now thought to be possible in hydrothermal systems (as high as 500°C at relevant pressures) and assessing the most extreme reaction conditions has thus far proven difficult. Metal and gas concentrations can easily reset upon conductive cooling during ascent to the seafloor. Dissolved silica concentrations have long been utilized as a geothermometer/barometer. It is possible, however, that silica values in high-T fluids may also reset during ascent, especially given the pressure and temperature sensitivity of quartz saturated fluids. Thus, silica values are highly dependent on in-situ fluid density and resultant electrolyte concentrations. It is therefore important to better assess the effects of near critical and two-phase conditions on the chemical evolution of hydrothermal fluids in order to differentiate between simultaneous and/or consecutive mineral equilibration and phase separation processes. Such data will aid in modeling efforts, which depend most highly on the breadth and quality of experimental data. In addition, these data will allow for broader interpretations of the growing database of hydrothermal fluid chemistry from mid-ocean ridges.
Reference:
Berndt, M. E., and W. E. Seyfried, Jr. (1993), Calcium and sodium exchange during hydrothermal alteration of calcic plagioclase at 400°C and 400 bars, Geochim. Cosmochim. Acta, 57, 4445-4451
Figures:
Figure 1. a) Na vs. Ca in high-T end-member fluids for several hydrothermal vent sites, including unpublished data from MEF, Lucky Strike and Rainbow. Superimposed lines represent plagioclase (An82 and An60) – quartz fluid equilibria at 400°C, 400 bars (after Berndt and Seyfried, 1993). Pester_fig1a.jpg b) Time series Ca/Cl vs. Na/Ca from four well studied vent fields. Solid line represents the charge balance Cl- = Na+ + 2Ca²+ for reference. Pester_fig1b.jpg See text for discussion.